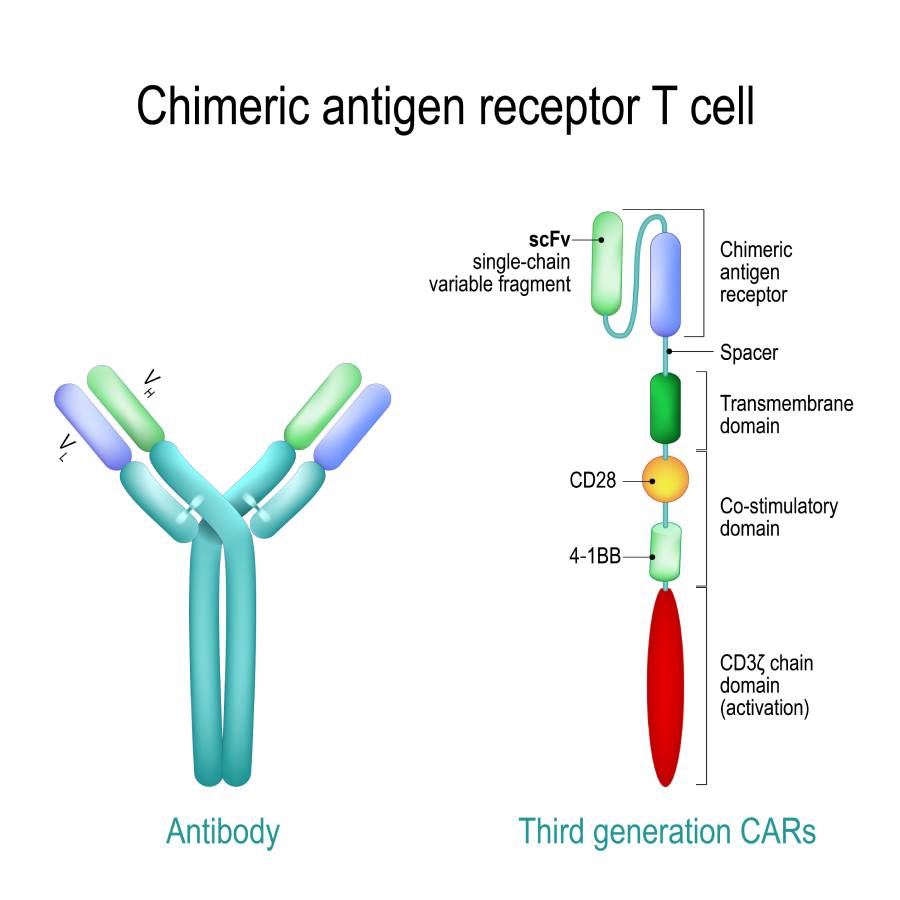
The implementation of novel immunotherapy agents has greatly altered the management and treatment of various malignancies. Many options are now available and are designed to target specific components of the immune system. Some of these novel therapies include immune checkpoint inhibitors, chimeric antigen receptor T cell therapies, cancer vaccines, and non-specific immunotherapies (1). In particular, immune checkpoint inhibitors (ICPs) have proven to be effective in a wide range of malignancies. Although historically reserved for late-stage metastatic cancers, ICPs are increasingly being utilized in earlier stages of disease. In certain forms of cancer, ICPs are often administered prior to surgical intervention as a means to decrease tumor burden and to potentially improve operative outcomes. However, although efficacious, these immunotherapy agents can lead to immune-mediated adverse effects in various organ systems. As an increasing number of patients receiving immunotherapy present for surgery and anesthesia, it is essential that anesthesiologists are familiar with these drugs and their side effects to reduce the risk of perioperative adverse events (2).
A common adverse effect of ICPs is endocrine organ dysfunction (adrenal gland, thyroid gland, pancreas). The adrenal gland plays a very important role in the regulation of blood pressure, acid-base homeostasis, and electrolyte balance. Therefore, adrenal gland insufficiency (secondary to immunotherapy usage) can manifest as electrolyte abnormalities (i.e. low sodium) or persistent hypotension. If a patient develops intraoperative hypotension unresponsive to fluids or vasopressors, adrenal insufficiency should be considered. In that case, a rescue dose of 100 mg of hydrocortisone IV should be administered, followed by continued supplementation of 50 mg of hydrocortisone IV every 6 h (2).
It is also important to pay attention to thyroid function tests in this patient population, as hypothyroidism is also a common side effect of immunotherapy. Anesthetic complications from hypothyroidism include delayed emergence, hypothermia, bradycardia, low cardiac output, and an impaired hypoxic and hypercapnic respiratory drive (2). In severe cases of hypothyroidism, it is recommended to administer either 200–400 μg of intravenous levothyroxine (T4) followed by 100 μg daily (2).
Along with adrenal and thyroid abnormalities, the pancreas may also be impaired by immunotherapy, with implications for the anesthesia and surgical teams. Although very rare, diabetes can be caused by immunotherapy-related pancreatic dysfunction. Thus, it is important to monitor for hyperglycemia throughout the perioperative period and administer insulin as necessary (2).
Pulmonary toxicity associated with immunotherapy may vary, but the most common complication is pneumonitis (inflammation of the lungs) (3). The clinical presentation of this condition characteristically involves the presence of a cough, shortness of breath, wheezing, and hypoxemia. Any pulmonary effects from immunotherapy require careful consideration due to the importance of respiratory function and airway patency during anesthesia. Management of pneumonitis requires the use of corticosteroids. In patients with a poor response to steroids, infliximab or cyclophosphamide may be needed. The judicious use of fluids and the use of protective ventilation strategies to reduce lung injury in this population is essential (3).
Last, but not least, there are also cardiac toxicities associated with immunotherapy patients that can impact the safety of surgery and anesthesia. These cardiotoxic effects may manifest as heart failure, rhythm abnormalities, myocarditis (inflammation of the myocardium) or pericarditis (inflammation of the pericardium). In the preoperative setting, anesthesiologists should review the patient’s ECG, cardiac enzyme levels, and echocardiogram to evaluate for any signs of cardiac dysfunction. Overt signs of heart failure should prompt anesthesiologists to postpone elective surgery until the patient is medically optimized. Otherwise, intraoperative management should include the use of invasive arterial pressure monitoring and intraoperative transesophageal echocardiography to monitor cardiac contractility and guide fluid management (2).
References
- Robinson AC, Wolfe RC. Immune Checkpoint Inhibitors and Perioperative Considerations. J Perianesth Nurs. 2020;35(6):687-691. doi:10.1016/j.jopan.2020.08.007
- Lewis AL, Chaft J, Girotra M, Fischer GW. Immune checkpoint inhibitors: a narrative review of considerations for the anaesthesiologist. Br J Anaesth. 2020;124(3):251-260. doi:10.1016/j.bja.2019.11.034
- Puzanov I, Diab A, Abdallah K, et al. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer. 2017;5(1):95. Published 2017 Nov 21. doi:10.1186/s40425-017-0300-z